20 September, 2020

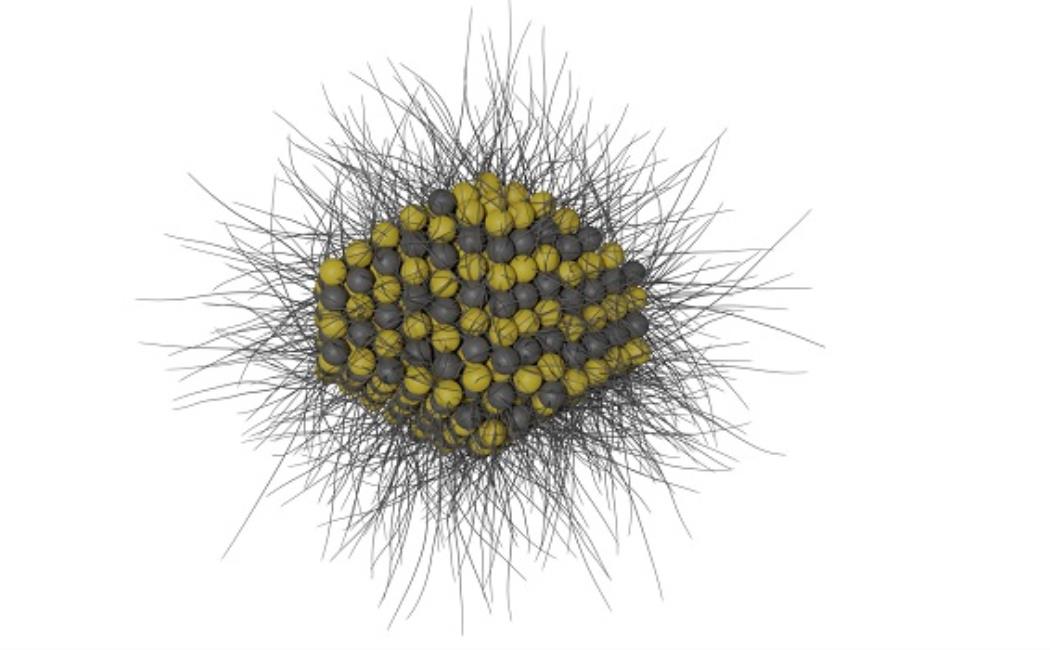
A lead sulfide quantum dot with long-chain surface ligands. Solar cells made with quantum dots show great promise as the next generation photovoltaic technology, but need to demonstrate long-term stability.
© KAUST 2020; Ahmad Kirmani
A process developed at KAUST for depositing extremely thin and smooth films can make it easier to manufacture stable solar cells based on quantum dot technology.
Colloidal quantum dots are tiny semiconductor particles capable of absorbing light over a broad range of wavelengths. Because these dots are easy to mix into liquid solvents, researchers have used them as "solar inks" that can be printed onto bendable plastic sheets. However, early prototypes revealed that exposure to air and ultraviolet radiation degraded the cell’s ability to transform sunlight into electricity.
“Prior to 2014, colloidal quantum dot solar cells were very unstable and could not survive outside a controlled nitrogen environment,” says KAUST alum Ahmad Kirmani. “This situation changed with the development of a new architecture that improved both device stability and power conversion efficiency.”
The latest quantum dot solar cells sandwich the tiny particles between two films referred to as either electron- or hole-transporting layers. These coatings are designed to quickly extract negative or positive charges generated by photoexcited dots to an external circuit. In addition, the layers provide much-needed protection against external elements.
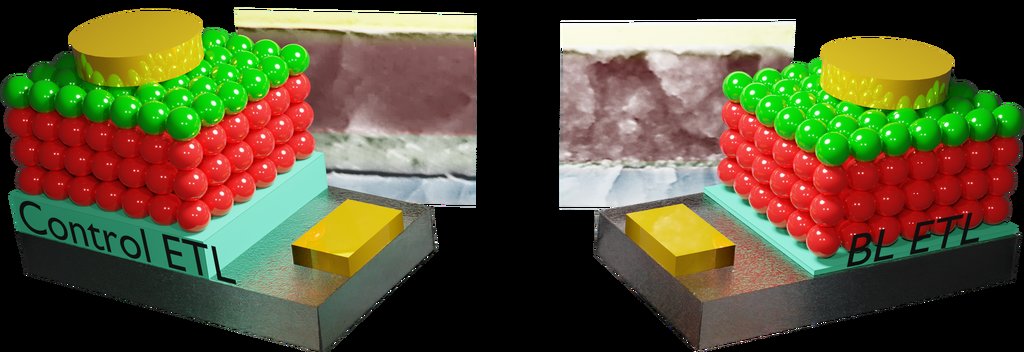
Schematic showing a control solar cell with a thick zinc oxide electron-transport layer (ETL) (left) and a solar cell employing the ultrathin and stable electron-transport layer developed in this work (right). SEM images are behind each schematic.
© KAUST 2020; Ahmad R. Kirmani
Kirmani and his colleagues realized that reducing the size of the electron-transporting layer could boost quantum dot solar cell performance. These films often comprise ultraviolet-sensitive materials, such as zinc oxide, and typically need to be more than 100-nanometers thick to prevent formation of defects that may short out the device. In contrast, thinner films are more desirable because they can extract photogenerated electrons at higher speeds.
The KAUST team developed a two-step technique to produce ultrathin films that are smooth enough for efficient electron collection. First, they deposited an indium oxide coating onto a transparent electrode to promote highly ordered film growth. A second deposition of zinc oxide, only 20-nanometers high, sealed up any porous defects and generated an extremely uniform interface.
“Initially, we struggled with device reproducibility due to surface irregularities,” says Kirmani. “Ultrathin films, however, adhere better to the substrate. By optimizing the solution concentrations, we relieved mechanical stresses to fabricate very flat films.”
Comparisons with a control device demonstrated that the ultrathin electron-transporting layer worked just as efficiently as a thicker zinc oxide film. Surprisingly, the mix of zinc and indium oxides in the new solar cell prolonged its shelf life, operational stability and tolerance to ultraviolet rays—advantages that the team attributes in part to enhanced optical transmittance through the device.